Publications
What are Extracellular Vesicles (EVs)? Subdividing Stem Cell Exosomes: Distinct Uses for Different Types
Do you know what "Extracellular Vesicles" (EVs) are? Many people encounter this term when first researching stem cell-derived exosomes or related applications and seek to understand its meaning. Simply stated, EVs can be regarded as "small delivery parcels" that facilitate intercellular communication; they comprise various apoptotic bodies, microvesicles, and exosomes. This article provide an in-depth examination of the differences between extracellular vesicles and exosomes.
What are Extracellular Vesicles (EVs)? Exploring the Differences Between EVs & Exosomes!
With the official enactment of Taiwan’s Regenerative Medicine Act and the Regenerative Medicinal Products Act (collectively referred to as Regenerative Medicine Dual Acts) in 2026, the application of cell therapies and cell derivatives has entered a new regulatory era. Among the various regenerative medicine technologies, extracellular vesicles (EVs) have received considerable attention within the field of regenerative medicine.
The legal term "cell derivatives" is a broad concept employed by the Taiwan Food and Drug Administration (TFDA) to encompass all products related to extracellular vesicles (EVs). When further subdivided, these products are commonly categorized as "exosomes" and "extracellular vesicles." In recent years, the term "exosomes" has become widely recognized, although a variety of technical terms (e.g., EVs, exosomes, vesicles, secretome) can often be overwhelming and confusing.
"Extracellular Vesicles" is the official regulatory term that has recently been standardized. It is defined as a composite group of cellular vesicles, including exosomes, microvesicles, and apoptotic bodies. Among these, microvesicles and apoptotic bodies are larger in size and possess biological functions that are distinct from those of exosomes.
From a purity and classification perspective, "exosomes" are a specific subset of the EV family. They are defined as nanosized vesicles within a specific size range and of high purity, generally between 30-150 nm. Following the recommendations of the International Society for Extracellular Vesicles (ISEV), the term “exosomes” has been formally adopted by many regulatory authorities worldwide.
Definitions and Sources: EVs vs. Exosomes
To establish a foundational concept, a simple approach to distinguish Extracellular Vesicles (EVs) & Exosomes is by their particle size hierarchy:
Comparison Table: Definitions and Characteristics
| Items | Extracellular Vesicles (EVs) | Exosomes |
| Official Name & Definition | The official term adopted in Taiwan; a collective term for all vesicles secreted by cells and may also be referred to as the secretome. | A specific category of vesicles within the EV family characterized by smaller particle size (30-150 nm) and specific conditions. |
| Composition | A broad mixture of all EV subtypes includes apoptotic bodies (50–5,000 nm), microvesicles (100–1,000 nm), and exosomes (30–150 nm). | The smallest subset of EVs, whose membrane biomarkers typically include CD9, CD63, and CD81. |
| Particle Size | Approximately 50 to 5,000 nanometers (nm). | Approximately 30 to 150 nanometers (nm). |
| Main Contents | Metabolites, cytokines, organelles, nucleic acids, proteins, lipids, etc. | Growth factors, nucleic acids, trophic factors, lipids, etc. |
| Application Fields | Generally investigated as biomarkers for disease diagnosis and monitoring, while certain EV-derived materials are used in cosmetic and beauty products. | Primarily applied in skincare and medical aesthetics, with some also being investigated or used in disease treatment. |
| Product Labeling | If labeled as "Extract" or "Extracellular Vesicles," they should be referred to as extracts or EVs. | If the material holds international certification (e.g., INCI name) and is designated as an "Exosome," the product can be formally labeled as "Exosome." |
| Purity & Regulation | Broad scope with significant variations in purity. | Clearer definition with higher requirements for purity and source. |
Analysis of Potential Applications: EVs versus Exosomes
| Item | Extracellular Vesicles (EVs) | Exosomes |
| Particle Characteristics | Wide size range; complex composition | Nano-sized vesicles (approx. 30 –150nm) with certain biomarkers expression (e.g., CD9, CD63, CD81). |
| Main Source | Includes apoptotic bodies and various vesicles. | Secreted by human or mammalian cells; commonly derived from Mesenchymal Stem Cells (MSCs). |
| Representative Components | Contains various disease-related indicators and diverse biomolecules. | Rich in trophic factors, growth factors, and nucleic acid fragments. |
| Common Applications | Disease diagnosis, beauty products. | Broad applications spanning medical aesthetics, skincare, regenerative medicine, and biomedical research. |
| Cosmetic Application | Plant-derived EVs can be used as raw materials for brightening, moisture, anti-inflammation, and antioxidant effects. | Certified exosome products are typically characterized by their reported ability to promote tissue repair, penetrate the skin barrier, and exert therapeutic effects. |
| Biological Interaction | Mainly focus on indicator (biomarker) analysis and component diversity. | Nanosized particles enable them to penetrate the Blood-Brain Barrier (BBB) or epidermal tissue, reaching deeper tissue layers to exert biological effects. |
| Research Potential | Diagnostics and basic researches. | International researches commonly highlights the therapeutic potential of exosomes in anti-inflammation, immunomodulation, wound healing, and regenerative repair. |
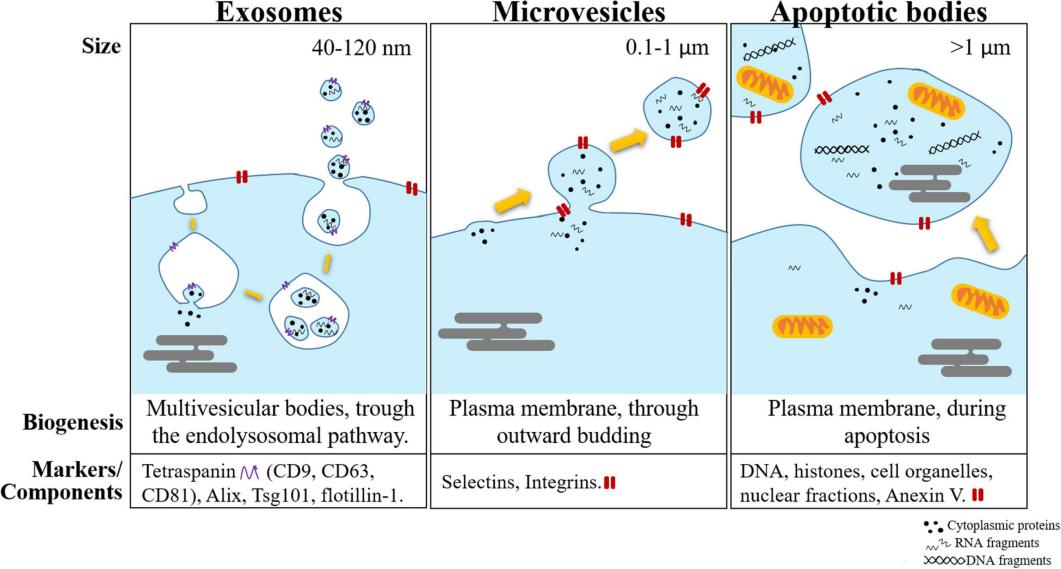
Figure 1: Mechanisms and Types of Extracellular Vesicle Biogenesis (Source: Marta Prieto-Vila et al., 2021)
Summary of Differences Between EVs and Exosomes
While the terms are often used interchangeably in the market, they are not identical. Their regulations, positioning, and applications different:
| Feature | Extracellular Vesicles (EVs) | Exosomes |
| Positioning | A collective term defined under cell derivatives. | Nano vesicles defined for drug development and high purity cosmetics; the official term recognized by international associations (ISEV), INCI, and regulatory bodies. |
| Particle Size | Covers all vesicles released by cells; size approx. 50–5000nm. | Focused specifically on nano-sized vesicles of 30–150nm. |
| Main Function | Metabolic waste removal and complext intercellular signaling. | Carry diverse bioactive signals and have been investigated for their ability to penetrate biological barriers and mediate biological effects in target tissues. |
| Common Applications | Diagnosis, cosmetic ingredients, and basic research. | Cosmetic ingredients, medical aesthetics, and regenerative medicine product (e.g., cell-free therapy, gene therapy) development. |
Functions and Applications of Exosomes
Exosomes are primarily secreted by mammalian cells (i.e., human cells). When they meet exosome specifications under high purity conditions, their overall application value and R&D potential are significantly higher. They have become a vital material in several industries:
- Regenerative Medicine & Adjunctive Therapy: Exosomes are widely used in cell-free therapy for treating osteoarthritis (OA), neurological injuries, and lung diseases. Unlike cell therapies, exosomes do not self-replicate, offering higher biological stability, lower immunogenicity, and high safety.
- Skin Repair & Medical Aesthetics: With a small size of 30–150 nm, exosomes can penetrate the skin barrier and interact with dermal fibroblasts, potentially promoting collagen production. They are commonly applied in skin regeneration, soothing, and post-procedural care.
- Early Diagnosis: By isolating exosomes from body fluids or blood and analyzing specific biomarkers, exosomes can be used for disease diagnosis (e.g., cancer).
Quality Control for EVs: Two Essential Standards
Quality Control (QC) is the key metric for ensuring safety and efficacy when translating research findings into clinical or commercial applications:
1. International Standards (MISEV Guidelines): Per MISEV 2023, clinical-grade exosomes must pass rigorous identification:
- Identity: Detection of at least three positive surface markers (e.g., Tetraspanins CD63, CD81, CD9) and at least one negative marker (e.g., Calnexin) to prove the absence of organelle contamination.
- Physical Characterization: Using Nanoparticle Tracking Analysis (NTA) to measure particle concentration (e.g., > 10^10 particles/mL) and size distribution. Transmission Electron Microscopy (TEM) is also recommended to confirm a complete lipid bilayer membrane and the cup-shaped structure.
2. Taiwanese Regulations & High Safety Testing: For human-derived EVs, manufacturers must follow TFDA regulations:
- Legal Sourcing: Proof of cell origin and donor screening reports to ensure the absence of pathogens like HIV, HBV, and CMV.
- Safety Verification: Batch wise testing for sterility, endotoxins, mycoplasma, and toxicity.
- Stability Testing: Data proving that particle number, size, protein concentration, and bioactivity remain consistent throughout the shelf life.
Stem Cell Derived Exosomes CDMO | Gwo Xi Stem Cell
Under the push of the Regenerative Medicine Dual Laws, Taiwan's CDMO industry is entering a golden age. Gwo Xi Stem Cell, with years of expertise in stem cell technology and clinical experience, is a leading company in Mesenchymal Stem Cell (MSC) exosome contract manufacturing in Taiwan. Utilizing its PIC/S GMP facilities and patented technologies, Gwo Xi can offer stable, large scale production of high-purity exosomes.
Technical Highlights:
- Modular Production Platform: Reduces human contamination risks and ensures batch-to-batch consistency in size and concentration.
- Patented Induction Technology: Uses patented media to enhance secretion efficiency and regulate levels of growth factors, miRNA, and trophic factors.
- Multiple Purification Technologies: Validated methods to isolate high-purity exosomes for everything from cosmetic-grade to clinical-grade.
Services and Achievements:
- Skincare Ingredient Supply: Providing exosome raw materials for high end cosmetics.
- Regenerative Medicine Product Development: Providing certified cell starting materials or producing cell derivatives to assist partners in process optimization and pre-clinical evaluation.
Gwo Xi Stem Cell offers a one-stop solution from R&D to commercialization. If you are interested in exosome raw material manufacturing, please Contact Us to start a new chapter in regenerative medicine!
References
- Théry, C., et al. (2018). Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. Journal of extracellular vesicles, 7(1), 1535750.
- Raposo, G., & Stoorvogel, W. (2013). Extracellular vesicles: exosomes, microvesicles, and friends. The Journal of cell biology, 200(4), 373–383. https://doi.org/10.1083/jcb.201211138
- Kalluri, R., & LeBleu, V. S. (2020). The biology, function, and biomedical applications of exosomes. Science (New York, N.Y.), 367(6478), eaau6977.
- Prieto-Vila, M., Yoshioka, Y., & Ochiya, T. (2021). Biological Functions Driven by mRNAs Carried by Extracellular Vesicles in Cancer. Frontiers in cell and developmental biology, 9, 620498.
